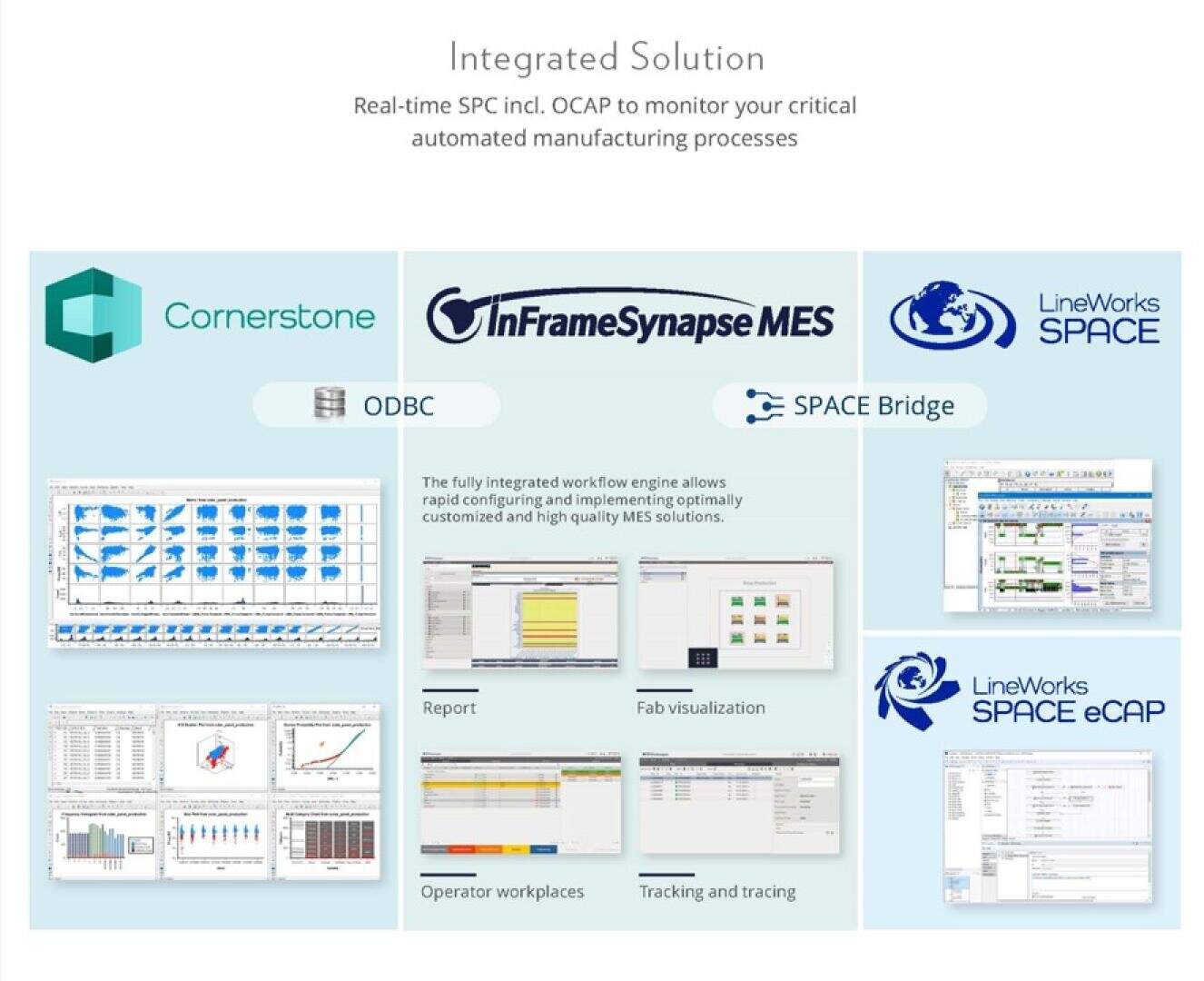
Synapse MES Medical Edition - the powerful Manufacturing Execution System that covers your entire production process!
Optimize your manufacturing processes in medical technology with camLine - IT solution partner to the high-tech for more than 30 years to increase productivity, quality and process integrity.
In a world where compliance, traceability, and quality assurance are of paramount importance, the medical devices industry requires cutting-edge solutions to meet the increasing healthcare demands.
On the other hand, digitalization in the medical devices production is still limited. From batch sheets to quality checks, tasks are still commonly done on paper. All this paperwork needs to be physically stored or converted into digital format, which can be time-consuming. Although it's currently the common practice, given the numerous steps and intricacy, paperwork tends to pile up and can easily become disorganized over time.
When the time comes for regulatory approval, certification, and audits, companies must provide the necessary documents on time to comply with the requirements. It gets more complicated when a Supplier Lot of a greater production chain needs to be tracked down in the case of a recall. Finding specific ones for the occasion can be time consuming and expensive if the data is not automatically and digitally stored.
With this in mind, can a properly digitalized system meet these challenges?
Corresponding to the current technological advancements, a digital solution covering the whole production process is the answer to this pressing question.
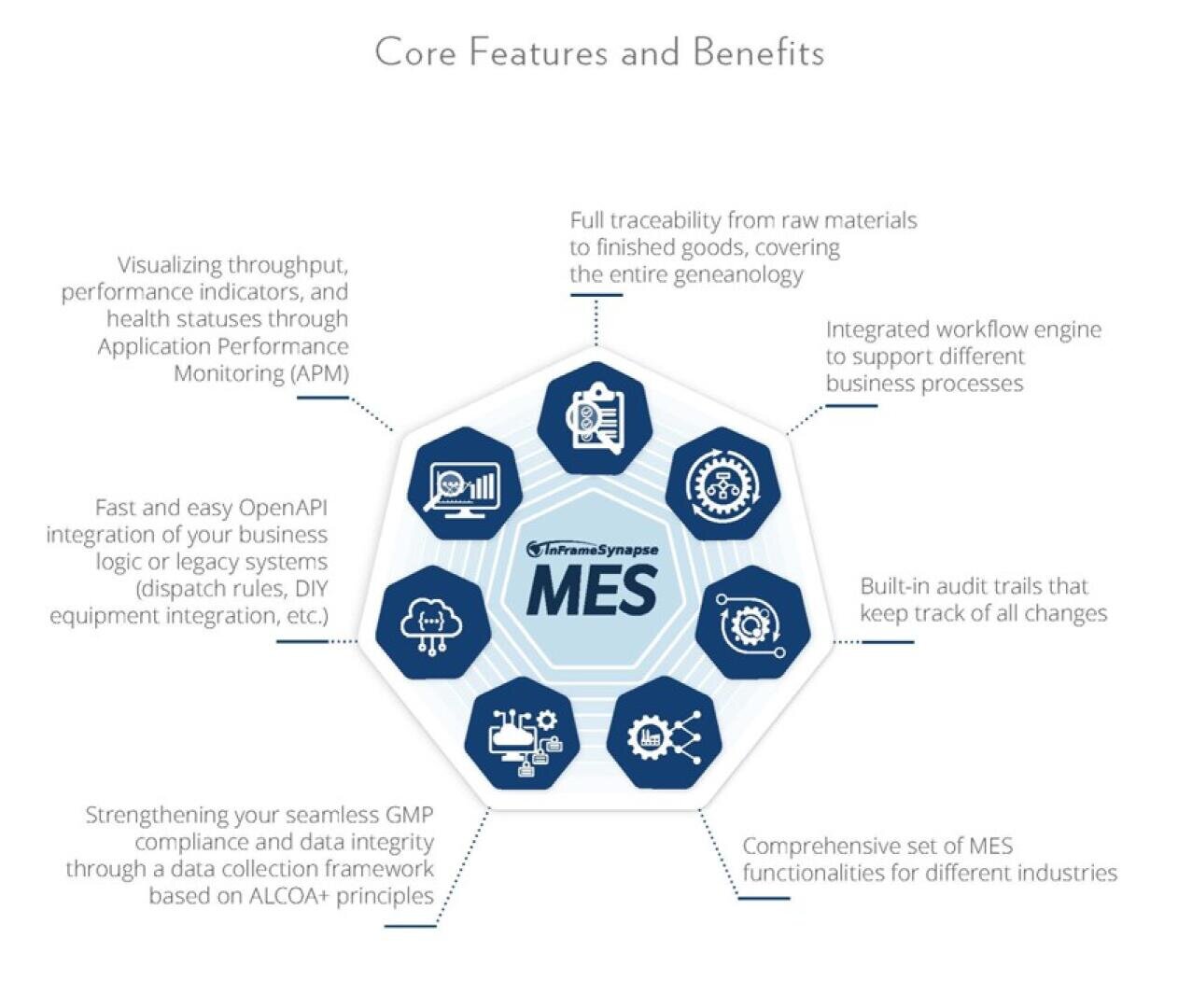
With the right solution, you can expect the following benefits:
- complete manufacturing process traceability from the start to finish,
- secured releasing and versioning quality and master data with electronic signature,
- secured and configurable Audit Trail,
- comprehensive track and trace system,
- easy configurable inspections,
- and Data integrity & Data ownership security.
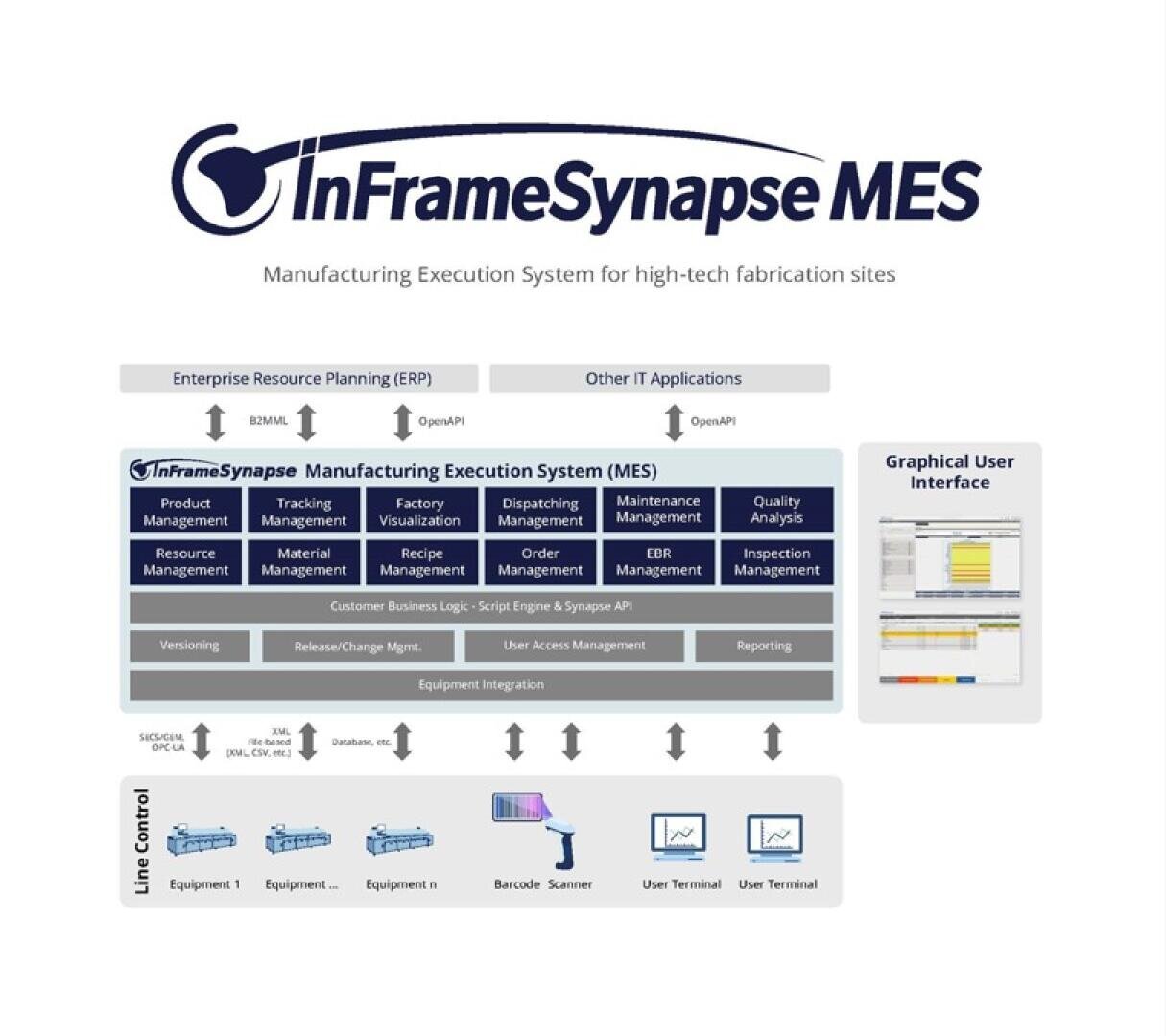
The powerful manufacturing execution system is an all-in-one discrete manufacturing execution solution. It ensures compliance, traceability, and quality control of product outputs, anytime and anywhere. It is easy to implement into your manufacturing system, helping you meet the standards for Good Manufacturing Practices within ISO 13485, 21 CFR Part 820, and other quality management system requirements.
It tracks every step of the manufacturing process, preventing errors, and ensures quality through real-time data and automated quality checks. With this, the products manufactured are up to the industry standard.
This solution facilitates an Electronic Batch Record (EBR) or Device History Record (DHR) , simplifying the production documentation process and traceability management. It streamlines operations, enhances efficiency, and ensures the safe production of medical devices.
The system is capable of documenting complete, consistent, and accurate data in accordance with the ALCOA++ documentation principles and with security checksum (SHA-2) against data manipulation. This guarantees full data integrity, meeting regulatory compliances, and seamless audits.
This system supports EBR, allowing you to electronically track and document your manufacturing lots and units with a fixed timestamp. In the case of a product recall, EBR lets you quickly identify the affected products and all involved Supplier Lots, streamline the recall process, and protect customers.
The user interface shows a list of any incidents that may have occurred during charge processing, with each incident requiring justification and an electronic signature that follows the CFR Part 11. It’s activated for runtime situations, such as scraps, inspections, carrier manipulation, and other changes.
Versioning ensures the traceability of changes throughout the manufacturing process - what was changed, when and why it got changed, who made the changes, and who approved it.
On top of that, the audit trail feature allows you to set up your own configurations for the user actions, activities, or data changes required to document on the database according to your processes. It lets you stay on track with the documentation for future audits.
Let’s be audit ready and reach your manufactory’s full potential!
Leverage your quality assurance, traceability, and efficiency with InFrame Synapse MES Medical Devices Edition!