Services for the CE certification of medical devices
CE Marking MDR (EU) 2017/745: Regulation (EU) 2017/745 on medical devices (EU Medical Device Regulation) is the medical device law that manufacturers must comply with in order to place medical devices on the European market.
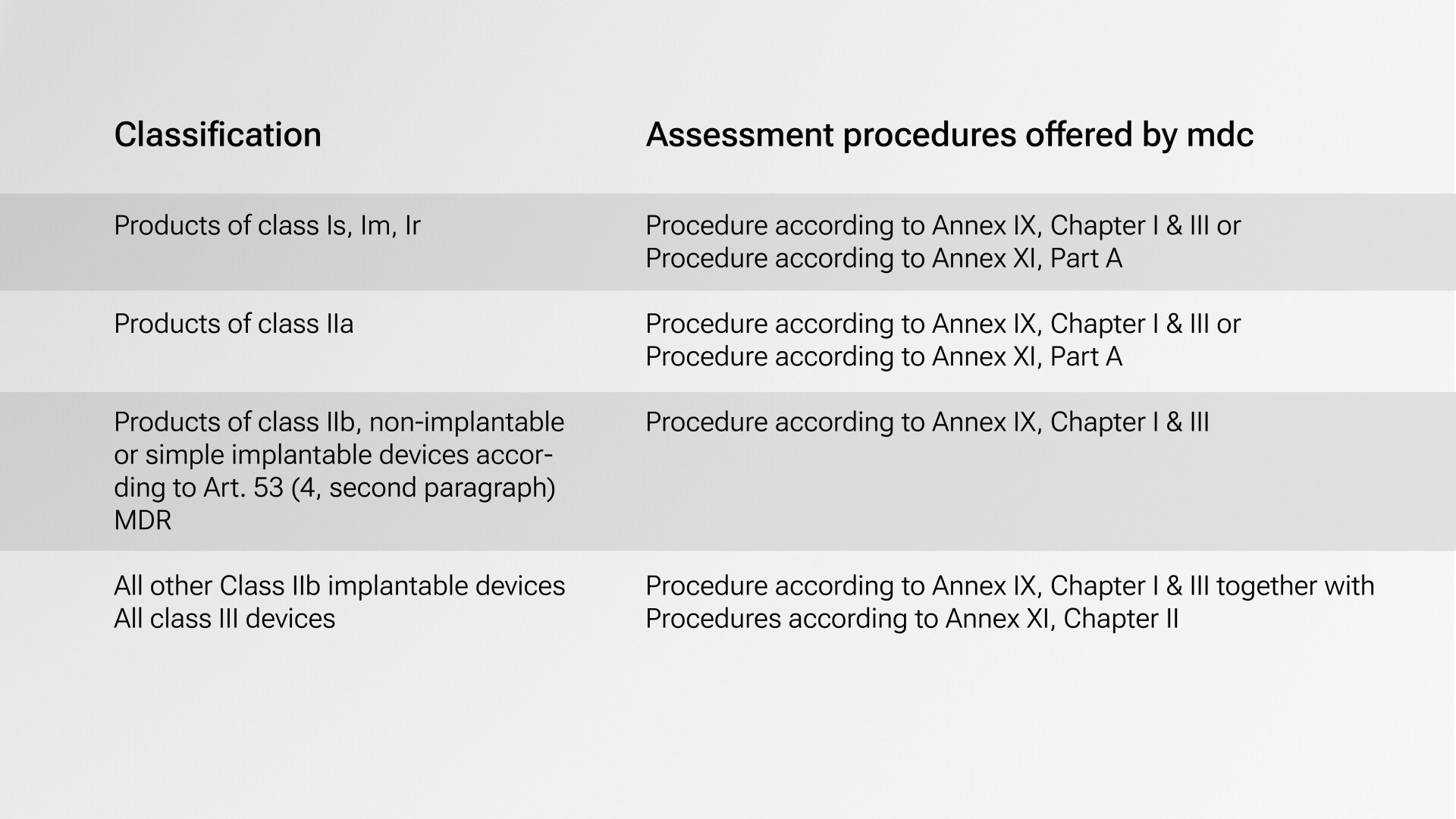
As a Notified Body, mdc is your competent partner for CE certification in accordance with the new Regulation (EU) 2017/745. The adoption of Regulation (EU) 2017/745 in the European Parliament brought numerous changes for the certification of medical devices. The MDR differentiates different risk classes I, I*, IIa, IIb and III for medical devices; the classification determines which conformity procedure is required and possible for market approval. Apart from the lowest risk class (class I), a notified body must be involved in the conformity assessment of all medical devices - to varying degrees, depending on the patient risk.
As the MDR is directly applicable law as a European regulation and replaces the previous directives (MDD and AIMDD), manufacturers must go through a complete re-assessment of all devices. Such a conformity assessment procedure under the MDR is equivalent to recertification and is mandatory for placing medical devices on the market in Europe.