Efficient Development of Medical Devices: A Ventilator Case Study Using CANoe
Medical devices play a critical role in healthcare, and their efficient development is essential to ensure patient safety and performance. In this article we delve into a real-world case study that demonstrates how the advanced software tool CANoe streamlines the development process of a ventilator.
Introduction
CANoe is an advanced software tool for the efficient development of medical devices. Designed to simplify the development, testing, and analysis of embedded systems, it supports system architects, network designers, developers, and test engineers throughout the entire development process – from planning to system-level tests. This concise case study demonstrates the application of CANoe in the development process of a ventilator.
The Ventilator System Under Test (SUT)
Our case study focuses on a ventilator that interacts with two components:
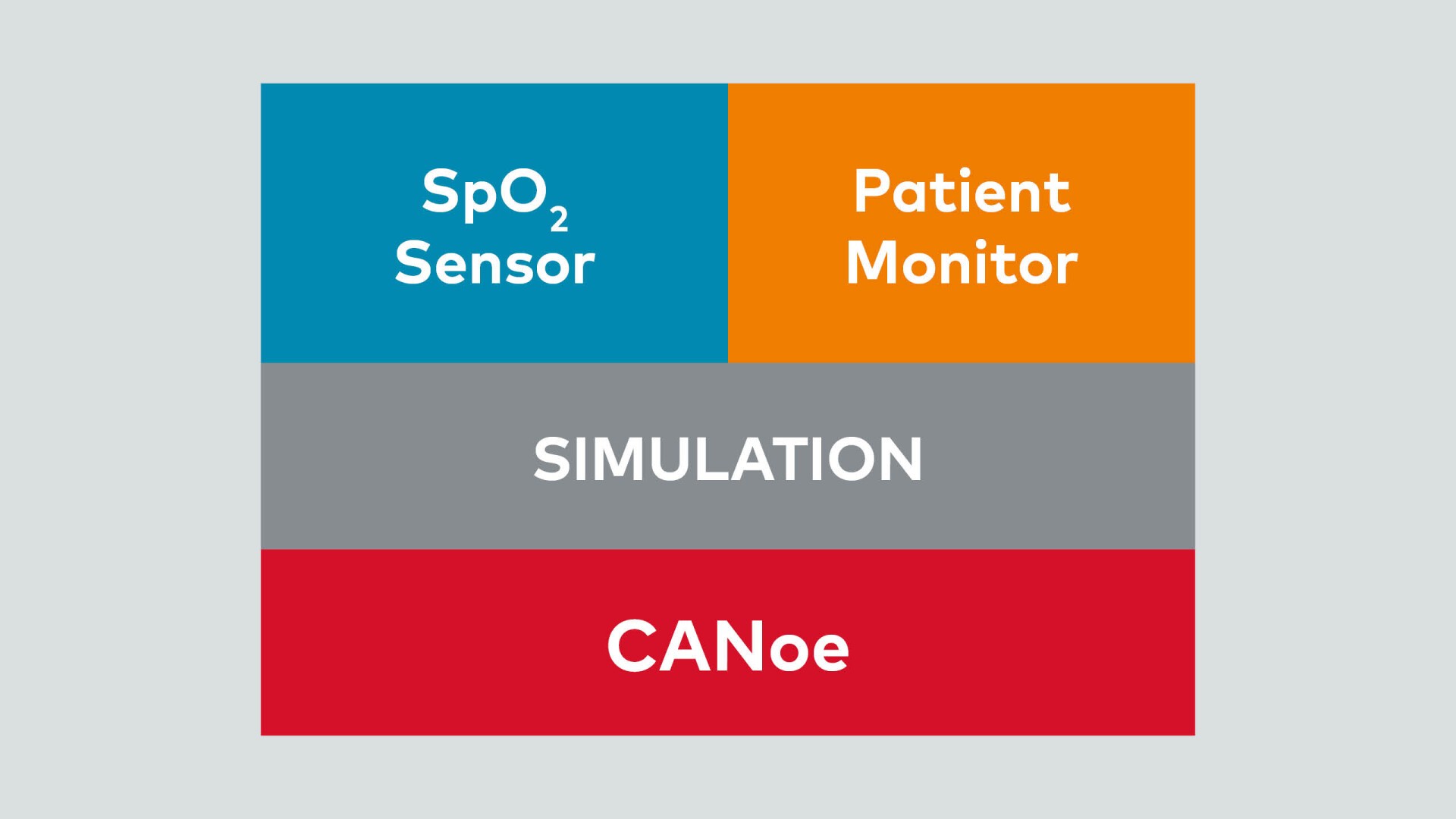
- SpO2 Sensor: This sensor provides the patient’s vital signs to the ventilator, including the pleth curve (a graphical representation of blood volume changes), oxygen saturation levels, and pulse rate. The communication between the SpO2 sensor and the ventilator occurs via a serial interface.
- Patient Monitor: The patient monitor receives ventilation parameters and alerts from the ventilator. These updates are transmitted using an Ethernet protocol.
The Three Stages of Development
The development of the ventilator involves a well-structured process, ensuring robustness, reliability, and adherence to requirements. Let's explore the three main stages:
1. Environment Simulation
During the initial development phase, CANoe steps in to simulate both the SpO2 sensor and the patient monitor (Image 02). This simulation serves a crucial purpose: to verify that the ventilator responds correctly to sensor inputs. By emulating realistic scenarios, CANoe ensures that the ventilator produces accurate outputs, which are then transmitted to the patient monitor. This step is essential for catching any early discrepancies and fine-tuning the system.
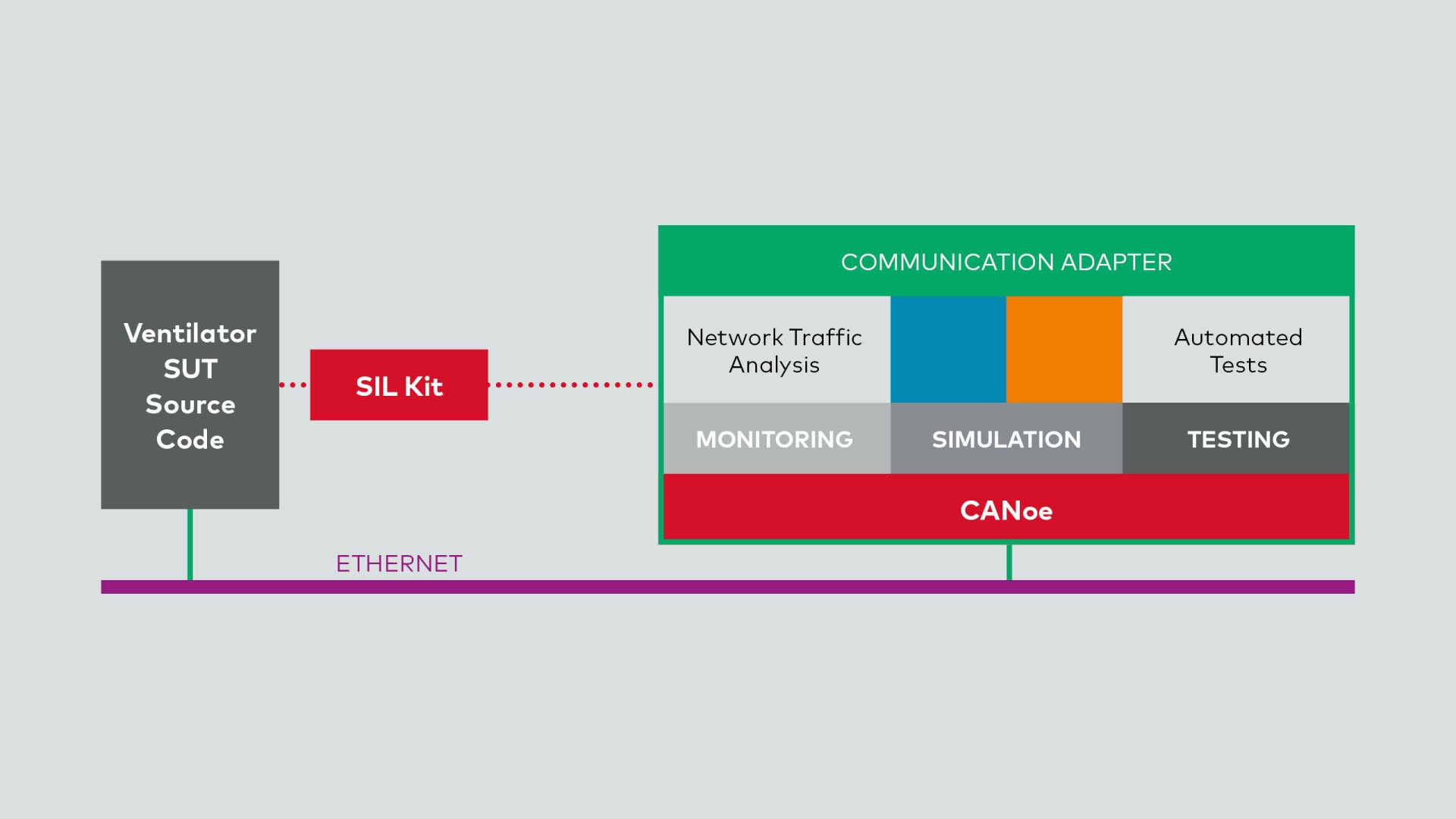
2. Software-in-the-Loop (SIL) Testing
With the ventilator's source code in place, it's time for SIL testing within CANoe. Here's how it works (Image 03):
- Stimulation: The software running on the ventilator is stimulated using the sensor simulation. The SIL Kit, an integral part of CANoe, simulates the serial connection. Inputs from the SpO2 sensor trigger responses from the ventilator software.
- Output Analysis: CANoe analyzes the outputs produced by the software via an actual Ethernet connection. Does the ventilator adjust its settings appropriately? Are alarms triggered correctly? Any deviations are flagged for further investigation.
- Automated Testing: Systematic and automated tests ensure that the software meets all specified requirements. Early bug detection significantly reduces development costs and prevents issues downstream.
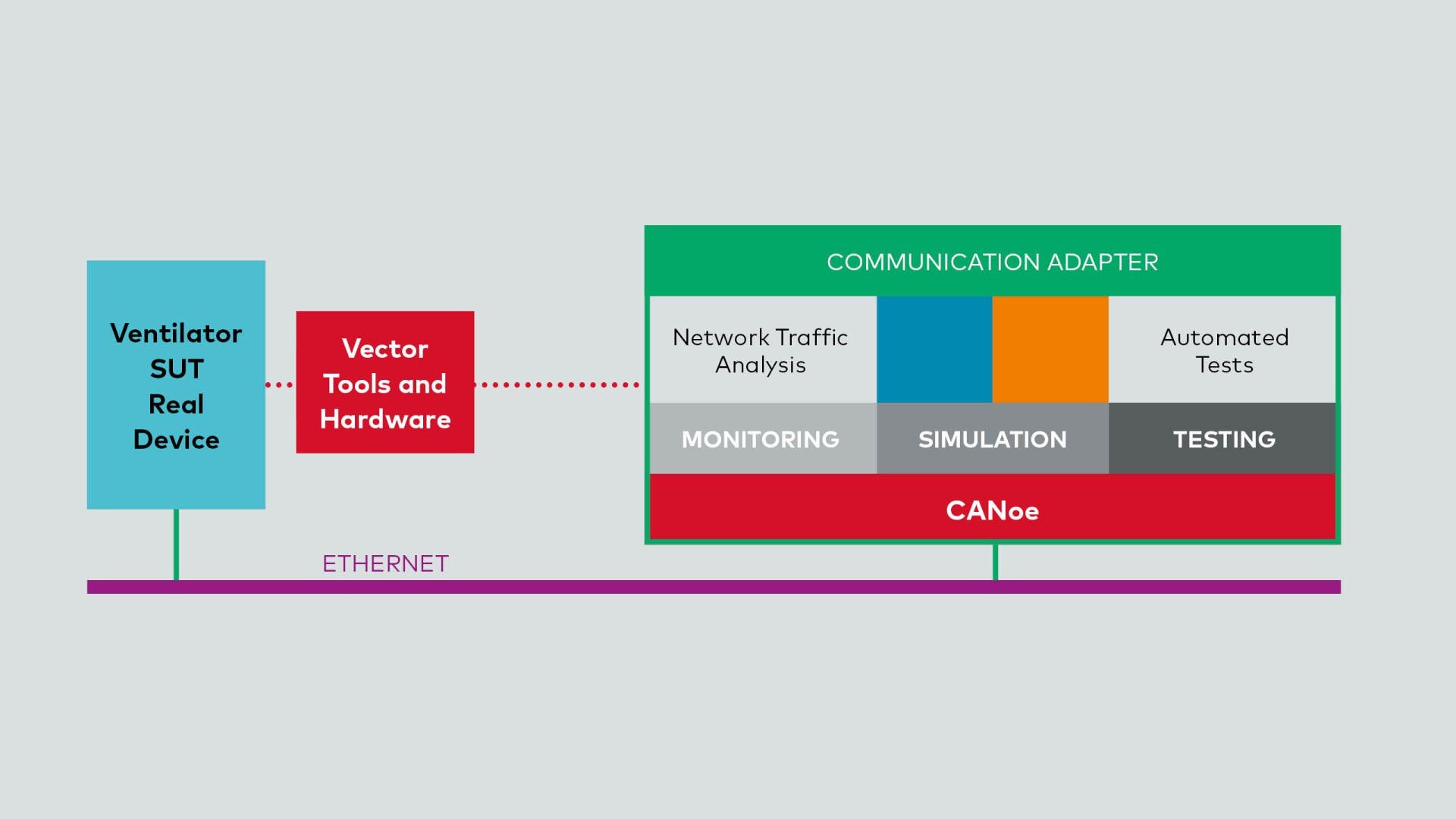
3. Hardware-in-the-Loop (HIL) Integration
Once the software passes SIL testing, it's time to integrate it into the physical hardware — the actual ventilator (Image 04). The HIL setup involves connecting the previously simulated serial interface to the real hardware interface. This transition from simulation to hardware-based testing allows for final verifications and adjustments. Engineers can fine-tune parameters, validate real-world behavior, and ensure that the ventilator performs optimally before deployment.
Conclusion
The ventilator case study exemplifies how CANoe accelerates the development of critical medical devices. By combining simulation, SIL testing, and HIL integration, engineers achieve efficiency, reliability, and confidence in their life-saving creations.
More about Vector Medical Engineering Solutions: medical.vector.com