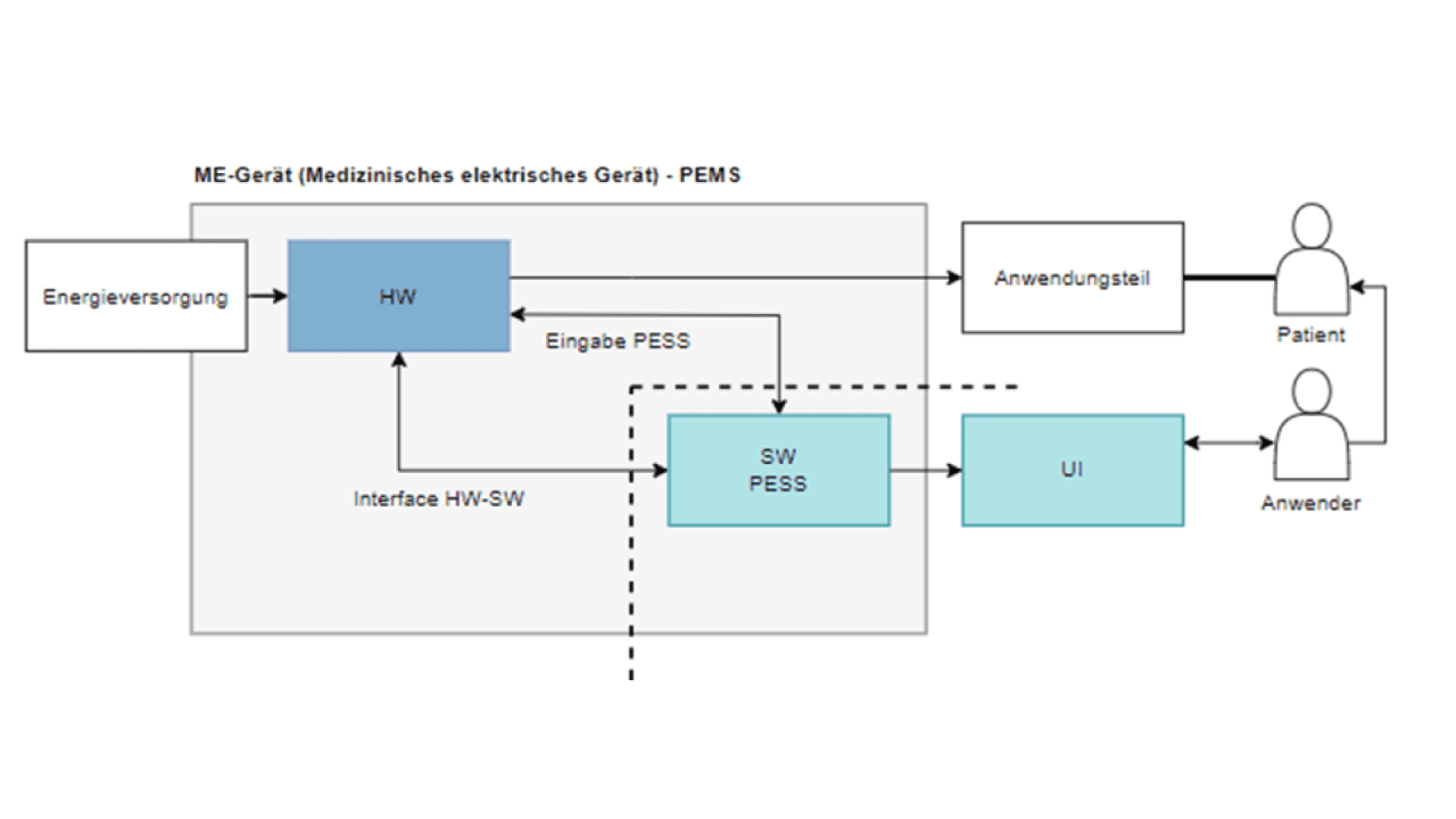
Risk-based system architecture
In its Appendix I the MDR requires the development of safe, effective medical products. During development, there's a risk of not specifying the product correctly or specifying it to an exaggerated extent. Our systematic approach to a risk-based system architecture provides a remedy and saves costs.
When determining the functional or systemic architecture of a medical product to be developed, it is important to coordinate system requirements, system architecture and risk management. If this approach is not pursued in a systematic manner, there is a risk of unnecessary effort and costs being incurred.
Pantec Biosolutions AG uses validated, software-supported tools to record system requirements, create the system architecture, the risk management as well as the usability. We support our customers and partners with a collaborative approach and ensure an effective start all the way up to product development and launch.